by P. Byrne1 (4/08)
Quick Facts…
- Bio-pharming is the production of pharmaceutical proteins in genetically engineered plants.
- Pharmaceuticals can be made in plants at a significantly reduced cost compared to current production methods.
- Major concerns with bio-pharming are that food or feed crops may become contaminated with pharmaceutical products, and that the products may have negative effects on natural ecosystems.
- Bio-pharm crops are regulated by two federal agencies (USDA and FDA) and by state departments of agriculture.
Manufacturing pharmaceutical products in crops has been one of the promised benefits of plant genetic engineering for the past 20 years. This use of biotechnology, sometimes known as “pharming,” “bio-pharming,” or “molecular farming,” has migrated from speculation to the
testing phase in fields and greenhouses across the country. Bio-pharming promises more plentiful and cheaper supplies of pharmaceutical drugs, including vaccines for infectious diseases and therapeutic proteins for treatment of such things as cancer and heart disease. “Plant-made pharmaceuticals” (PMPs) are produced by genetically engineering plants to produce specific compounds, generally proteins, which are extracted and purified after harvest. As used here, the terms bio-pharming and PMP do not include naturally occurring plant products or nutritionally enhanced foods.
How are biotech drugs currently manufactured?
Many protein-based drugs are currently produced in sterile fermentation facilities by genetically engineered microorganisms or mammalian cell cultures in stainless steel tanks (Felsot, 2002). Because these fermentation plants have huge capital construction costs, industry has been unable to keep up with the growing demand. For example, the biotech company Amgen is reportedly unable to meet demand for Enbrel, a protein-based arthritis medicine made in mammalian cell cultures (Alper, 2003). Another method for obtaining biopharmaceuticals is to extract them from animal and human tissues (e.g., insulin from pig and cow pancreas, or blood proteins from human blood (Freese, 2002)). However, these are high-cost procedures that carry the risk of transmitting infectious diseases to humans. Due to advances in plant genetic engineering over the past two decades, plants can now be modified to produce a wide range of proteins. It is hoped this will result in therapeutic products at a price significantly cheaper than through current methods. For example, antibodies that currently cost thousands of dollars per gram might be produced in plants for $200 per gram (Ohlrogge and Chrispeels, 2003).
What pharmaceuticals could be made in plants?
For the near-term, PMPs will be proteins because proteins are directly encoded by genes. This property makes their production through genetic engineering more straightforward than other types of biochemical compounds that are synthesized via more complex methods. See Table 1 for examplesof potential bio-pharm products.
| Table 1. Potential plant-made pharmaceuticals. Information compiled from Canadian Food Inspection Service (2001), Ohlrogge and Chrispeels (2003), and the Web sites of the Biotechnology Industry Organization (www.bio.org), and Ventria Bioscience (www.ventria.com). | ||
| Product | Definition | Examples |
| Antibodies | Specialized proteins of the immune system that initiate the body’s defense response. |
Specific antibodies may be developed to fight cancer, HIV-AIDS, hepatitis, malaria, dental caries, and other diseases. |
| Antigens (vaccines) | Compounds that elicit the production of antibodies that protect against disease. |
Plant-made vaccines are currently under development for protection against cholera, diarrhea (Norwalk virus), and hepatitis B. |
| Enzymes | Proteins that catalyze biochemical reactions. | Enzymes could be used both to treat and to diagnose disease. For example, lipase is an enzyme that breaks down dietary fats and is used to treat symptoms of cystic fibrosis and other diseases. |
| Hormones | Chemical messengers active at low concentrations and produced in specialized cells. |
Insulin is produced in the pancreas and helps regulate sugar metabolism. Diabetics with insulin deficiencies must replace it via shots or pumps. |
| Structural proteins | Proteins that provide structural supportto cells or tissues. | Collagen is a structural protein found in animal connective tissues and used in cosmetics. |
| Anti-disease agents | A wide variety of proteins. | The anti-infection proteins interferon and lactoferrin, and aprotinin (which controls blood loss during surgery) have been engineered in plants. |
What crops are being considered for pharmaceutical production?
The most common PMP crops grown in U.S. field trials are corn, tobacco, and rice. Other crops being investigated include alfalfa, potato, safflower, soybean, sugarcane, and tomato. Suitable host plants must be easily engineered, capable of high levels of protein production, and have appropriate procedures for extracting the PMP from plant tissues. Knowing the agronomy, physiology, pests, and diseases of a crop is also an advantage. Ideally, the host plant is a non-food crop that does not have wild relatives in the production environment, and could not survive in the environment from seeds carried by wind or wildlife. When food crops are used, complete pollen sterility is desired, since it would prevent nearby fields of the crop from being pollinated by the bio-pharm crop. While this is not yet technically feasible, using self-pollinating crops or male-sterile crops can minimize pollination of food crops.
What part of the plant produces the PMP?
Most bio-pharming applications target production and storage of the pharmaceutical protein in seeds, which naturally accumulate high concentrations of proteins and oils. Seeds are also the easiest part of the plant to store and transport to processing facilities. To achieve production of
the pharmaceutical protein in seeds, seed-specific “promoters” (regulatory elements of genes that control how much of a gene product is made and where in the plant it is synthesized) are engineered into the plant. Two seed-specific promoters used in experimental bio-pharm lines are the beta-phaseolin promoter of common bean and the oleosin promoter of Brassica species (Moloney, 2000). The location of protein accumulation within the cell is also important to ensure correct folding and stability of the protein (Moloney, 2000).
While synthesis of biopharmaceuticals in seeds has many advantages, not all PMPs will be produced there. Leaves are the target tissues in some alfalfa and tobacco applications, and tubers are targeted in potato production systems (Canadian Food Inspection Service, 2001). A variation of PMP technology involves infecting plants with viruses that are engineered with the gene for the pharmaceutical protein. Upon infection (for example, of tobacco leaves), the plant’s cellular machinery produces the biopharmaceutical along with other viral proteins (Freese, 2002).
How will PMPs be produced?
To be successful, pharmaceutical production in plants must be a highly sophisticated and closely regulated enterprise, and will differ from conventional crop production in many ways. Bio-pharm crops must be grown, transported, and processed using safeguards designed to ensure a consistent, high-quality product and to prevent inadvertent mixing with food crops and other negative consequences. To achieve this goal, a “closed loop identity preservation” system is envisioned, in which the crop is carefully regulated and monitored from planting to harvest to pharmaceutical extraction (Felsot, 2002). Seed will be available only to trained contract growers, and the harvested product will be delivered in sealed containers to the processing facility.
Standard operating procedures developed for each specific PMP crop will govern isolation distances from conventional crops, equipment use, and field inspections during the growing season and for at least a year afterward. Meticulous record-keeping will be required at each step of the process.
When will plant-made pharmaceuticals reach the market?
Research on PMP crops has been ongoing for many years in laboratories, greenhouses, and field trials. In 2002, PMP crops were grown at 34 field sites totaling 130 acres in the U.S. Three PMPs currently undergoing evaluation in clinical trials are designed to target non-Hodgkins lymphoma, cystic fibrosis, and E. coli/traveler’s diarrhea (Biotechnology Industry Organization). Assuming their effectiveness and safety are demonstrated and environmental concerns are adequately addressed, therapuetic pharmaceuticals from plants may reach the market in the second half of this decade.
Who is doing bio-pharming?
Among the companies pursuing commercial development of PMPs are Dow AgroSciences, Meristem Therapeutics, and Ventria Bioscience. Reflecting the commercial uncertainty of the industry, some companies, including Monsanto, have discontinued development of PMP products, and the biopharmaceutical firms CropTech and Large Scale Biology Corp. have filed for bankruptcy in recent years. The companies that develop PMPs will most likely contract with a limited number of highly skilled farmers to produce bio-pharm crops.
What are the benefits of plant-made pharmaceuticals?
- PMPs can be produced at a significantly reduced cost compared to current production methods. Therefore, the technology has the potential to benefit medical patients by providing a more affordable source of vaccines and other pharmaceuticals. However, it is not clear how large the cost reduction will be or how much of the savings will be passed on to consumers.
- Plants can be engineered to produce proteins of greater complexity than is possible with microorganisms (Collins, 2003), and to produce proteins that cannot be produced in mammalian cell cultures (Anonymous, 2002).
- A limited number of growers and production workers will likely benefit economically from this new agricultural enterprise. The number of acres required to produce a year’s worth of a given pharmaceutical will likely be quite small compared to crop acreage for food and feed use.
What are the risks of plant-made pharmaceuticals?
Risks are not uniform for all bio-pharm applications, but will vary depending on the nature of the pharmaceutical product, the crop and tissues in which the PMP is produced, and the environment in which the crop is grown. The potential risk factors of PMPs are summarized below.
- Pollen from plants engineered to produce pharmaceuticals may fertilize nearby food or feed crops of the same species. If this occurs, the pharmaceutical may be produced in seed of the neighboring crop, with potentially negative effects on human or animal consumers of the seed and on crop markets. The risk of gene flow via pollen drift is greater in cross-pollinated crops like corn. Methods to minimize this risk include spatial and temporal isolation, the use of male sterility (i.e., plants that don’t produce viable pollen), and in the case of corn, detasseling (removing tassels before they shed pollen). When male sterility or detasseling are used, fertile male plants that lack the gene for the pharmaceutical are planted in the field to provide the pollen source.
- Commingling of PMP crops and food or feed crops may occur. This could happen through improper labeling, mixing of seed in planting, harvesting, transportation, or processing equipment, or the presence of “volunteer” PMP plants in subsequent seasons in the same field. In a 2001 case, ProdiGene failed to eliminate volunteer bio-pharm corn plants from a soybean crop planted later in the same field as the PMP corn (Fox, 2003). The company was fined $250,000 by USDA and was required to reimburse the government $3 million for expenses related to destruction of 500,000 bushels of potentially contaminated soybeans.
- The introduced gene or its product may have negative effects on the natural environment. For example, wildlife feeding on the crop may ingest harmful levels of the PMP, or soil micro-organisms may be inhibited by decomposing crop residue or substances exuded from roots of PMP plants.
- Farm workers may be exposed to unhealthy levels of a biopharmaceutical by absorbing products from leaves through their skin, inhaling pollen, or breathing in dust at harvest.
- Unexpected toxins or residues of pesticides used on the crop may contaminate the final drug product.
What steps are being taken to prevent or reduce these risks?
 |
All workers involved with PMP crop production must participate annually in an APHIS-approved training program on the required procedures for growing these crops. Photo courtesy of USDA. |
 |
Equipment for planting and harvesting of bio-pharm crops must be dedicated to that purpose, i.e., the equipment cannot be used with any other crop. Photo courtesy of USDA. |
 |
Tractors and tillage equipment must be thoroughly cleaned before being used with other crops. Photo courtesy of USDA. |
 |
Bio-pharmed fields will be closely monitored during the growing season and in following seasons to ensure that required procedures are being followed and that volunteer plants are found and disposed of properly. Photo courtesy of USDA. |
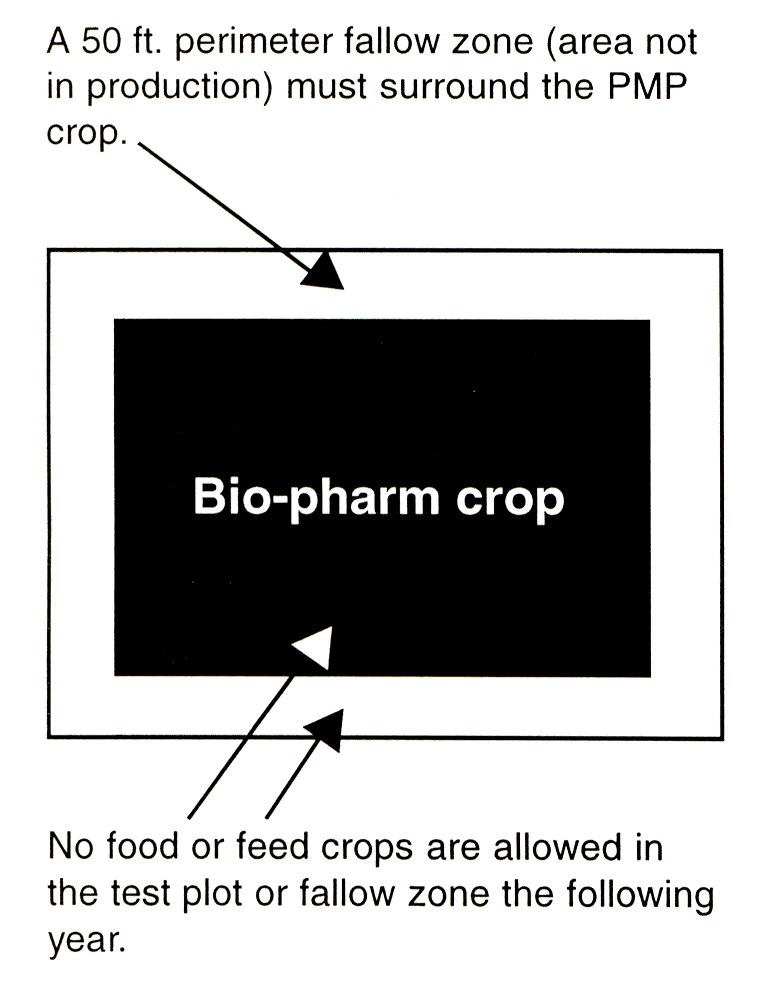 |
Test sites must provide the required isolation distances from other fields of the same crop. For example, bio-pharm corn must be isolated by at least 1 mile from other corn fields if it is open-pollinated, and by 1/2 mile if pollination is controlled through male sterility or detasseling. |
Because bio-pharm crops are genetically engineered, they are subject to U.S. federal regulations that govern all such crops. Three federal agencies, the U.S. Department of Agriculture – Animal and Plant Health Inspection Service (APHIS), the Food and Drug Administration (FDA), and the Environmental Protection Agency (EPA) all play roles in regulating genetically engineered crops, though their specific responsibilities vary depending on the type of application involved.
Besides the standard regulations that apply to all genetically engineered crops, bio-pharm crops are subject to additional regulatory oversight. One major difference between PMP crops and enetically engineered food crops is that the former require perpetual permitting by APHIS, whereas the latter crops, once approved by the three federal agencies, are considered “unregulated” and are freely available through commercial channels without permits. In September, 2002, FDA and USDA issued the draft document “Guidance for Industry: Drugs, Biologics, and Medical Devices Derived from Bioengineered Plants for Use in Humans and Animals”, https://www.federalregister.gov/documents/2002/09/12/02-23105/draft-guidance-for-industry-drugs-biologics-and-medical-devices-derived-from-bioengineered-plants. In 2003, APHIS announced its intentions to impose more stringent conditions for field tests of genetically engineered crops that produce pharmaceutical or industrial compounds. The objective of the new regulations is to prevent contamination of food and feed crops with the biopharmaceuticals and to minimize environmental impacts. In 2004, APHIS provided additional guidance for bio-pharm permit applicants (APHIS, 2004). For example, applicants are requested to provide details on the amount of the gene product in all plant parts, the results of allergenicity testing, and an assessment of potential toxicity to non-target organisms.
FDA has the responsibility to ensure the safety and usefulness of drugs. Therefore, clinical trials and marketing of PMPs will require FDA approval. FDA will also oversee procedures for manufacturing PMPs to guarantee consistent product quality and potency.
EPA regulates the environmental effects of proteins engineered for pest resistance (such as Bt insecticidal proteins) in a PMP crop. However, EPA does not review environmental effects of bio-pharm crops at this time.
The department of agriculture of the state in which a PMP crop field test is proposed, is given the opportunity to review APHIS’ preliminary assessment of applications for field testing of genetically engineered crops. In the past, this has been a routine approval, but with PMP crops, states are taking a more cautious approach. State departments of agriculture may request additional permit conditions beyond those imposed by APHIS.
Are bio-pharm crops likely to be grown in Colorado?
The advantages Colorado has for bio-pharming are the ease of achieving recommended isolation distances for many crops, and the ability to obtain high yields under irrigated conditions with relatively little disease and insect pest pressure.
The French company Meristem Therapeutics applied to APHIS for a permit to grow a field test of PMP corn in Phillips County, Colorado in 2003. To assist with evaluation of this and future permit applications for PMP crops, the Colorado Department of Agriculture (CDA) formed a Technical
Advisory Committee to evaluate the adequacy of conditions for gene containment and for minimizing environmental impact. Although the Meristem application was approved, the company decided not to plant the trial because the optimum planting date had passed.
In 2004, APHIS and CDA approved an application from an Iowa State University researcher to grow bio-pharm corn, and a small field plot was planted in Logan County. Whether commercial scale bio-pharm production will occur in Colorado depends on a number of business and government policy decisions, the outcomes of which are difficult to predict at present.The Colorado Institute of Public Policy has examined the issues involved in bio-pharming and published the report “Bio-Pharming in Colorado: A Guide to Issues for Making Informed Choices” (https://mountainscholar.org/handle/10217/185447).
Final Thoughts
Like many other aspects of crop biotechnology, supporters and critics of PMP crops differ strongly over the benefits and risks of this new application. Proponents stress the societal benefits of a cheaper and more plentiful source of pharmaceuticals, while opponents emphasize the risks of contamination of the food supply and unknown effects on ecosystems. Given the uncertainties
surrounding bio-pharm crops, it is difficult to predict whether and to what extent this technology will become part of our future agricultural and health care systems. Several questions remain to be answered, including: (1) Are PMPs safe and effective medicines for humans and animals? (2) Will production costs of PMPs, especially for the purification process, be reduced sufficiently to bring the promised economic benefits? (3) What will be the appropriate combinations of crop species, plant parts, growing environments, and production safeguards that will provide acceptable levels of gene containment and environmental protection? (4) Are our regulatory structures adequate to the task of regulating and monitoring bio-pharm crops, and, if not, what changes will be necessary? (5) To what extent will crop-based pharmaceuticals provide new economic opportunities for farmers and rural communities?
References
Alper, J. 2003. Hatching the golden egg: A new way to make drugs. Science 300:729-730.
Anonymous, 2002. Pharming the Field: A look at the benefits and risks of bioengineering plants to produce pharmaceuticals. The Pew Charitable Trusts. www.pewtrusts.org/our_work_report_detail.aspx?id=35424.
APHIS, 2003. USDA strengthens 2003 permit conditions for field testing genetically engineered plants. Press release. https://www.aphis.usda.gov/brs/fedregister/BRS_20030806a.pdf.
APHIS. 2004. Updated guidance on bioengineered plants for producing pharmaceuticals or industrial products for applicants developing these plants for release (January 2004).
Canadian Food Inspection Service. 2001. Plant molecular farming discussion document.
Collins, S. 2003. Potato the medical factory of tomorrow. The New Zealand Herald, April 4, 2003.
Felsot, A. 2002. Pharm farming. Agrichemical and Environmental News, no. 195, July 2002, http://aenews.wsu.edu.
Fox, J.L. 2003. Puzzling industry response to Prodigene fiasco. Nature Biotechnology 21: 3-4.
Freese, B. 2002. Manufacturing drugs and chemical crops: Biopharming poses new threats to consumers, farmers, food companies and the environment.
https://www.jstor.org/stable/resrep00065.6#metadata_info_tab_contents.
Moloney, M.M. 2000. Molecular farming using seeds as hosts. pp. 226-253. M. Black and J.D.Bewley (eds.) Seed technology and its biological basis. CRC Press, Boca Raton, FL.
Ohlrogge, J., and M.J. Chrispeels. 2003. Plants as chemical and pharmaceutical factories. pp. 500-527. M.J. Chrispeels and D.E. Sadava (eds.) Plants, genes, and biotechnology. Jones and Bartlett Publishers, Sudbury, MA.
Websites for Additional Information
The Union of Concerned Scientists https://www.ucsusa.org/resources/protecting-food-supply-era-pharmaceutical-and-industrial-crops discusses benefits and risks of pharm crops.
The Biotechnology Industry Organization, https://archive.bio.org/articles/plant-made-pharmaceuticals-background-and-key-points, has a number of fact on plant-made pharmaceuticals.
The Transgenic Crops website, http://cls.casa.colostate.edu/TransgenicCrops/what.html,
contains introductory information on the techniques and regulation of plant genetic engineering.
1 P. Byrne, Colorado State University Extension agronomy specialist and associate professor, department of soil and crop sciences. 7/03. Revised 4/08.
Colorado State University, U.S. Department of Agriculture and Colorado counties cooperating. CSU Extension programs are available to all without discrimination. No endorsement of products mentioned is intended nor is criticism implied of products not mentioned.
Go to top of this page.





