by T.A. Bauder, R.M. Waskom, P.L. Sutherland and J. G. Davis* (10/14)
Quick Facts…
- Knowledge of irrigation water quality is critical to understanding management for long-term productivity.
- Irrigation water quality is evaluated based upon total salt content, sodium and specific ion toxicities.
- In many areas of Colorado, irrigation water quality can influence crop productivity.
 |
Corn plant damaged by saline sprinkler water. |
Salt-affected soils develop from a wide range of factors including: soil type, field slope and drainage, irrigation system type and management, fertilizer and manuring practices, and other soil and water management practices. In Colorado, perhaps the most critical factor in predicting, managing, and reducing salt-affected soils is the quality of irrigation water being used. Besides affecting crop yield and soil physical conditions, irrigation water quality can affect fertility needs, irrigation system performance and longevity, and how the water can be applied. Therefore, knowledge of irrigation water quality is critical to understanding what management changes are necessary for long-term productivity.
Irrigation Water Quality Criteria
Soil scientists use the following categories to describe irrigation water effects on crop production and soil quality:
- Salinity hazard – total soluble salt content
- Sodium hazard – relative proportion of sodium to calcium and magnesium ions
- pH – acid or basic
- Alkalinity – carbonate and bicarbonate
- Specific ions: chloride, sulfate, boron, and nitrate.
Another potential irrigation water quality impairment that may affect suitability for cropping systems is microbial pathogens.
| Table 1. General guidelines for salinity hazard of irrigation water based upon conductivity. | |
| Limitations for use | Electrical Conductivity |
| (dS/m)* | |
| None | ≤0.75 |
| Some | 0.76 – 1.5 |
| Moderate1 | 1.51 – 3.00 |
| Severe2 | ≥3.00 |
| *dS/m at 25ºC = mmhos/cm1Leaching required at higher range.2Good drainage needed and sensitive plants may have difficulty at germination. | |
Salinity Hazard
The most influential water quality guideline on crop productivity is the water salinity hazard as measured by electrical conductivity (ECw). The primary effect of high ECw water on crop productivity is the inability of the plant to compete with ions in the soil solution for water (physiological drought). The higher the EC, the less water is available to plants, even though the soil may appear wet. Because plants can only transpire “pure” water, usable plant water in the soil solution decreases dramatically as EC increases.
The amount of water transpired through a crop is directly related to yield; therefore, irrigation water with high ECw reduces yield potential (Table 2). Actual yield reductions from irrigating with high EC water varies substantially. Factors influencing yield reductions include soil type, drainage, salt type, irrigation system and management. Beyond effects on the immediate crop is the long term impact of salt loading through the irrigation water. Water with an ECw of only 1.15 dS/m contains approximately 2,000 pounds of salt for every acre foot of water. You can use conversion factors in Table 3 to make this calculation for other water EC levels.
| Table 2. Potential yield reduction from saline water for selected irrigated crops.1 |
||||
| % yield reduction | ||||
| Crop | 0% | 10% | 25% | 50% |
| ECw2 | ||||
| Barley | 5.3 | 6.7 | 8.7 | 12 |
| Wheat | 4.0 | 4.9 | 6.4 | 8.7 |
| Sugarbeet3 | 4.7 | 5.8 | 7.5 | 10 |
| Alfalfa | 1.3 | 2.2 | 3.6 | 5.9 |
| Potato | 1.1 | 1.7 | 2.5 | 3.9 |
| Corn (grain) | 1.1 | 1.7 | 2.5 | 3.9 |
| Corn (silage) | 1.2 | 2.1 | 3.5 | 5.7 |
| Onion | 0.8 | 1.2 | 1.8 | 2.9 |
| Dry Beans | 0.7 | 1.0 | 1.5 | 2.4 |
| 1Adapted from “Quality of Water for Irrigation.” R.S. Ayers. Jour. of the Irrig. and Drain. Div., ASCE. Vol 103, No. IR2, June 1977, p. 140. 2ECw = electrical conductivity of the irrigation water in dS/m at 25oC. 3Sensitive during germination. ECw should not exceed 3 dS/m for garden beets and sugarbeets. |
||||
Other terms that laboratories and literature sources use to report salinity hazard are: salts, salinity, electrical conductivity (ECw), or total dissolved solids (TDS). These terms are all comparable and all quantify the amount of dissolved “salts” (or ions, charged particles) in a water sample. However, TDS is a direct measurement of dissolved ions and EC is an indirect measurement of ions by an electrode.
Although people frequently confuse the term “salinity” with common table salt or sodium chloride (NaCl), EC measures salinity from all the ions dissolved in a sample. This includes negatively charged ions (e.g., Cl–, NO–3) and positively charged ions (e.g., Ca++, Na+). Another common source of confusion is the variety of unit systems used with ECw. The preferred unit is deciSiemens per meter (dS/m), however millimhos per centimeter (mmho/cm) and micromhos per centimeter (µmho/cm) are still frequently used. Conversions to help you change between unit systems are provided in Table 3.
| Table 3. Conversion factors for irrigation water quality laboratory reports. | |||
| Component | To Convert | Multiply By | To Obtain |
| Water nutrient or TDS | mg/L | 1.0 | ppm |
| Water salinity hazard | 1 dS/m | 1.0 | 1 mmho/cm |
| Water salinity hazard | 1 mmho/cm | 1,000 | 1 µmho/cm |
| Water salinity hazard | ECw (dS/m) for EC <5 dS/m |
640 | TDS (mg/L) |
| Water salinity hazard | ECw (dS/m) for EC >5 dS/m |
800 | TDS (mg/L) |
| Water NO3N, SO4-S,B applied | ppm | 0.23 | lb per acre inch of water |
| Irrigation water | acre inch | 27,150 | gallons of water |
Definitions |
|
| Abbrev. | Meaning |
| mg/L | milligrams per liter |
| meq/L | milliequivalents per liter |
| ppm | parts per million |
| dS/m | deciSiemens per meter |
| µS/cm | microSiemens per centimeter |
| mmho/cm | millimhos per centimeter |
| TDS | total dissolved solids |
Sodium Hazard
Infiltration/Permeability Problems
Although plant growth is primarily limited by the salinity (ECw) level of the irrigation water, the application of water with a sodium imbalance can further reduce yield under certain soil texture conditions. Reductions in water infiltration can occur when irrigation water contains high sodium relative to the calcium and magnesium contents. This condition, termed “sodicity,” results from excessive soil accumulation of sodium. Sodic water is not the same as saline water. Sodicity causes swelling and dispersion of soil clays, surface crusting and pore plugging. This degraded soil structure condition in turn obstructs infiltration and may increase runoff. Sodicity causes a decrease in the downward movement of water into and through the soil, and actively growing plants roots may not get adequate water, despite pooling of water on the soil surface after irrigation.
The most common measure to assess sodicity in water and soil is called the Sodium Adsorption Ratio (SAR). The SAR defines sodicity in terms of the relative concentration of sodium (Na) compared to the sum of calcium (Ca) and magnesium (Mg) ions in a sample. The SAR assesses the potential for infiltration problems due to a sodium imbalance in irrigation water. The SAR is mathematically written below, where Na, Ca and Mg are the concentrations of these ions in milliequivalents per liter (meq/L). Concentrations of these ions in water samples are typically provided in milligrams per liter (mg/L). To convert Na, Ca, and Mg from mg/L to meq/L, you should divide the concentration by 22.9, 20, and 12.15 respectively.
For most irrigation waters encountered in Colorado the standard SAR formula provided above is suitable to express the potential sodium hazard. However, for irrigation water with high bicarbonate (HCO3) content, an “adjusted” SAR (SARADJ) can be calculated. In this case, the amount of calcium is adjusted for the water’s alkalinity, is recommended in place of the standard SAR (see pH and Alkalinity section below). Your laboratory may calculate an adjusted SAR in situations where the HCO3 is greater than 200 mg/L or pH is greater than 8.5.
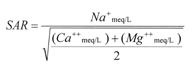 |
meq/L = mg/L divided by atomic weight of ion divided by ionic charge (Na+=23.0 mg/meq, Ca++=20.0 mg/meq, Mg++=12.15 mg/meq) |
The potential soil infiltration and permeability problems created from applications of irrigation water with high “sodicity” cannot be adequately assessed on the basis of the SAR alone. This is because the swelling potential of low salinity (ECw) water is greater than high ECw waters at the same sodium content (Table 4). Therefore, a more accurate evaluation of the infiltration/permeability hazard requires using the electrical conductivity (ECw) together with the SAR.
| Table 4. Guidelines for assessment of sodium hazard of irrigation water based on SAR and ECw2. | ||
| Potential for Water Infiltration Problem | ||
| Irrigationwater SAR | Unlikely | Likely |
| ———–ECw2 (dS/m)———- | ||
| 0-3 | >0.7 | <0.2 |
| 3-6 | >1.2 | <0.4 |
| 6-12 | >1.9 | <0.5. |
| 12-20 | >2.9 | <1.0 |
| 20-40 | >5.0 | <3.0 |
| 2Modified from R.S. Ayers and D.W. Westcot. 1994. Water Quality for Agriculture, Irrigation and Drainage Paper 29, rev. 1, Food and Agriculture Organization of the United Nations, Rome. | ||
Many factors including soil texture, organic matter, cropping system, irrigation system and management affect how sodium in irrigation water affects soils. Soils most likely to show reduced infiltration and crusting from water with elevated SAR (greater than 6) are those containing more than 30% expansive (smectite) clay. Soils containing more than 30% clay include most soils in the clay loam, silty clay loam textural classes and finer and some sandy clay loams. In Colorado, smectite clays are common in areas with agricultural production.
| Table 5. Susceptibility ranges for crops to foliar injury from saline sprinkler water. | ||||
| Na or Cl concentration (mg/L) causing foliar injury | ||||
| Na concentration | <46 | 46-230 | 231-460 | >460 |
| Cl concentration | <175 | 175-350 | 351-700 | >700 |
| Apricot | Pepper | Alfalfa | Sugarbeet | |
| Plum | Potato | Barley | Sunflower | |
| Tomato | Corn | Sorghum | ||
| Foliar injury is influenced by cultural and environmental conditions. These data are presented only as general guidelines for daytime irrigation. Source: Mass (1990) Crop salt tolerance. In: Agricultural Assessment and Management Manual. K.K. Tanji (ed.). ASCE, New York. pp. 262-304. | ||||
pH and Alkalinity
The acidity or basicity of irrigation water is expressed as pH (< 7.0 acidic; > 7.0 basic). The normal pH range for irrigation water is from 6.5 to 8.4. Abnormally low pH’s are not common in Colorado, but may cause accelerated irrigation system corrosion where they occur. High pH’s above 8.5 are often caused by high bicarbonate (HCO3–) and carbonate (CO32–) concentrations, known as alkalinity. High carbonates cause calcium and magnesium ions to form insoluble minerals leaving sodium as the dominant ion in solution. As described in the sodium hazard section, this alkaline water could intensify the impact of high SAR water on sodic soil conditions. Excessive bicarbonate concentrates can also be problematic for drip or micro-spray irrigation systems when calcite or scale build up causes reduced flow rates through orifices or emitters. In these situations, correction by injecting sulfuric or other acidic materials into the system may be required.
Chloride
Chloride is a common ion in Colorado irrigation waters. Although chloride is essential to plants in very low amounts, it can cause toxicity to sensitive crops at high concentrations (Table 6). Like sodium, high chloride concentrations cause more problems when applied with sprinkler irrigation (Table 6). Leaf burn under sprinkler from both sodium and chloride can be reduced by night time irrigation or application on cool, cloudy days. Drop nozzles and drag hoses are also recommended when applying any saline irrigation water through a sprinkler system to avoid direct contact with leaf surfaces.
| Table 6. Chloride classification of irrigation water. |
|
| Chloride (ppm) | Effect on Crops |
| Below 70 | Generally safe for all plants. |
| 70-140 | Sensitive plants show injury. |
| 141-350 | Moderately tolerant plants show injury. |
| Above 350 | Can cause severe problems. |
| Chloride tolerance of selected crops. Listing in order of increasing tolerance: (low tolerance) dry bean, onion, carrot, lettuce, pepper, corn, potato, alfalfa, sudangrass, zucchini squash, wheat, sorghum, sugar beet, barley (high tolerance). Source: Mass (1990) Crop Salt Tolerance. Agricultural Salinity Assessment and Management Manual. K.K. Tanji (ed.). ASCE, New York. pp 262-304. | |
Boron
Boron is another element that is essential in low amounts, but toxic at higher concentrations (Table 7). In fact, toxicity can occur on sensitive crops at concentrations less than 1.0 ppm. Colorado soils and irrigation waters contain enough B that additional B fertilizer is not required in most situations. Because B toxicity can occur at such low concentrations, an irrigation water analysis is advised for ground water before applying additional B to irrigated crops.
| Table 7. Boron sensitivity of selected Colorado plants (B concentration, mg/ L*) | ||||
| Sensitive | Moderately Sensitive | Moderately Tolerant | Tolerant | |
| 0.5-0.75 | 0.76-1.0 | 1.1-2.0 | 2.1-4.0 | 4.1-6.0 |
| Peach | Wheat | Carrot | Lettuce | Alfalfa |
| Onion | Barley | Potato | Cabbage | Sugar beet |
| Sunflower | Cucumber | Corn | Tomato | |
| Dry Bean | Oats | |||
| Source: Mass (1987) Salt tolerance of plants. CRC Handbook of Plant Science in Agriculture. B.R. Cristie (ed.). CRC Press Inc. *Maximum concentrations tolerated in soil water or saturation extract without yield or vegetative growth reductions. Maximum concentrations in the irrigation water are approximately equal to these values or slightly less. |
||||
Sulfate
The sulfate ion is a major contributor to salinity in many of Colorado irrigation waters. As with boron, sulfate in irrigation water has fertility benefits, and irrigation water in Colorado often has enough sulfate for maximum production for most crops. Exceptions are sandy fields with <1 percent organic matter and <10 ppm SO4-S in irrigation water.
Nitrogen
Nitrogen in irrigation water (N) is largely a fertility issue, and nitrate-nitrogen (NO3-N) can be a significant N source in the South Platte, San Luis Valley, and parts of the Arkansas River basins. The nitrate ion often occurs at higher concentrations than ammonium in irrigation water. Waters high in N can cause quality problems in crops such as barley and sugar beets and excessive vegetative growth in some vegetables. However, these problems can usually be overcome by good fertilizer and irrigation management. Regardless of the crop, nitrate should be credited toward the fertilizer rate especially when the concentration exceeds 10 ppm NO3-N (45 ppm NO3¯). Table 3 provides conversions from ppm to pounds per acre inch.
Summary
The quality of irrigation water available to farmers and other irrigators has a considerable impact on what plants can be successfully grown, the productivity of these plants, and water infiltration and other soil physical conditions. The first step in understanding how an irrigation water source can affect a soil-plant system is to have it analyzed by a reputable lab. The Colorado State University Extension factsheet, Selecting an Analytical Laboratory 0.520 can help you locate a lab in your area that is familiar with irrigation water quality. Additional information on understanding and managing for saline and sodic conditions is found in Colorado State University factsheets, Managing Saline Soils 0.503 and Managing Sodic Soils 0.504.
* T.A. Bauder, Colorado State University Extension water quality specialist; R.M. Waskom, director, Colorado Water Institute; P.L. Sutherland, USDA/NRCS area resource conservationist; and J.G. Davis, Extension soils specialist and professor, soil and crop sciences. 7/03. Revised 10/14.
Go to top of this page.





