by J.L. Smith and J.P. Workman * (11/14)
Revised by A. Drenth and P. Cabot**
Quick Facts…
- Alcohols burn more completely then petroleumbased fuels, thus increasing combustion efficiency.
- Advantages of mixing alcohol with gasoline are that alcohol tends to increase the octane rating and reduce carbon monoxide and other tailpipe emissions.
- There are many disadvantages to using alcohols, particularly methyl and ethyl alcohol.
- Alcohols may corrode certain materials used in engines.
Alcohol has been used as a fuel for internal combustion engines since their invention. Reports on the use of alcohol as a motor fuel were published in 1907 and detailed research was conducted in the 1920s and 1930s. Historically, the level of interest in using alcohol as a motor fuel has followed cycles of petroleum-based fuel shortages and/or low feed-grain prices. More recently, the use of alcohol and other renewable fuels has been driven by governmental mandates to reduce engine emissions and increase renewable fuel use. Ethyl alcohol (ethanol) is the primary domestically produced renewable fuel in the U.S. today. Currently, most U.S. ethanol production is from corn, where the starch in the corn kernel is converted to ethanol. Ethanol can also be made from lignocellulose, a structural material that comprises much of the mass of plants. Ethanol produced in this manner is known as cellulosic ethanol and can use corn stover, grasses, wood chips, and other plant materials as feedstock.
The properties of methyl, ethyl, and butyl alcohol are compared with octane (high quality gasoline) and hexadecane (high quality diesel fuel) in Table 1. Note that octane and hexadecane (petroleum fuels) have higher boiling points, lower latent heats, and are insoluble in water. The alcohols become more like petroleum fuels as their chemical weights increase.
Methyl alcohol has the lowest combustion energy of all the fuels listed. However, it also has the lowest stoichiometric or chemically correct air-fuel ratio. Therefore, an engine burning methyl alcohol would produce the most power. It also is possible to take advantage of the higher octane ratings of methyl (and ethyl) alcohol and increase the engine compression ratio. Increases in compression ratio increase the efficiency of converting the potential combustion energy to power. Finally, alcohols burn more completely, thus increasing combustion efficiency.
Disadvantages of Alcohol
There are many disadvantages to using alcohols, particularly methyl and ethyl alcohol. Although these alcohols, when used near their stoichiometric air-fuel ratios, produce more power, a larger quantity of fuel is required to produce a specified power output. For example, in an automobile, more fuel is required for each mile driven. Since the price of alcohol and conventional fuels both fluctuate, miles per dollar should be an important metric in which fuel type or blend percentage to use. Using alcohol or gasoline-alcohol blends generally reduces fuel economy (miles per gallon), but if the alcohol is cheaper, the economics (miles per dollar) may still be favorable.
The relatively low boiling points and high vapor pressures of methyl and ethyl alcohol indicate that vapor lock could be a serious problem, particularly at high altitudes on warm summer days. Vapor lock occurs when the liquid fuel changes state to a gas while still in the fuel delivery system. Vapor lock can cause reduce engine power or stalling. Butyl alcohol, because of its low vapor pressure, is the least likely of the alcohols to cause vapor lock.
The relatively high latent heats of methyl and ethyl alcohol cause problems in mixing these alcohols with air and transporting them through the intake manifold of the engine. Heating the intake manifold may be necessary in cold weather or before the engine reaches operating temperatures. Without external heat to more completely vaporize the fuel, the engine may be difficult to start and sluggish for a considerable time after starting. Butyl alcohol is the least likely to cause starting difficulties or problems during warm-up. Note that its latent heat is almost the same as the latent heat of octane.
All of the alcohols are soluble in water, but butyl alcohol is relatively insoluble compared to methyl and ethyl alcohol. Less engine power is produced as the water content of an alcohol increases. Further, vapor lock, fuel mixing, and starting problems increase with water.
| Table 1: Characteristics of chemically pure fuels. | |||||||||
| Chemical formula | Chemical weight (lb/mole) | Specific gravity | Boiling point (C) | Latent heat (Btu/lb) | Combustion energy (Btu/lb) | Vapor pressure @100F (psi) | Solubility part in 100 parts H2O | Stoichio-metric air-fuel ratio | |
| Methyl alcohol | CH3OH | 32 | 0.79 | 65 | 503 | 10,260 | 4.6 | infinite | 6.5 |
| Ethyl alcohol | CH3CH2(OH) | 46.1 | 0.79 | 78 | 396 | 13,160 | 2.2 | infinite | 9 |
| Butyl alcohol | C2H5CH2CH2(OH) | 74.1 | 0.81 | 117 | 186 | 15,770 | 0.3 | 9 | 11.2 |
| Octane | C8H18 | 114 | 0.70 | 210 | 155 | 20,750 | 1.72 | insoluble | 15.2 |
| Hexa-decane | C16H34 | 240 | 0.79 | 287 | — | 20,320 | 3.46 | insoluble | 15 |
| *To convert to metrics, use the following conversion factors: 1 pound = 45 kilogram; 1 degree F = degrees C – 32 x 5/9. |
|||||||||
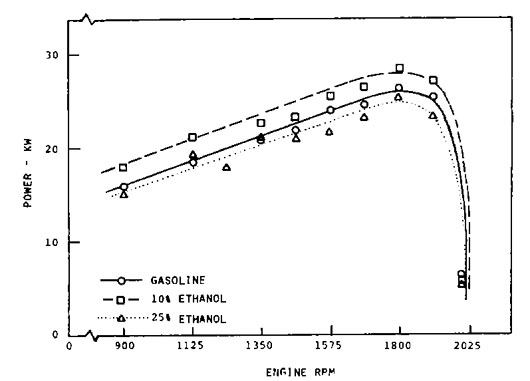
Figure 1: Gasoline engine full throttle power output using ethanol fuel blends. |
Blending Alcohol and Gasoline
Mixing alcohol with gasoline produces gasohol. Advantages of fuel blends are that alcohol tends to increase the octane rating and reduce carbon monoxide (CO) and other tailpipe emissions from the engine. The octane number of a fuel indicates its resistance to knock (abnormal combustion in the cylinder). Another advantage is that alcohols can also be produced from renewable sources.
The primary disadvantage of mixing methyl and ethyl alcohol with gasoline is that under certain conditions these alcohols may separate from the gasoline. An engine adjusted to burn gasoline efficiently will produce less power from alcohol should it separate from the gasoline. Separation is caused by the polar nature of the alcohol molecules and their tendency to absorb water, also a polar substance. Methyl alcohol is the most likely to separate while butyl alcohol is the least likely. The tendency for separation increases as the temperature decreases, the quantity of water absorbed increases, and the quality of the gasoline decreases.
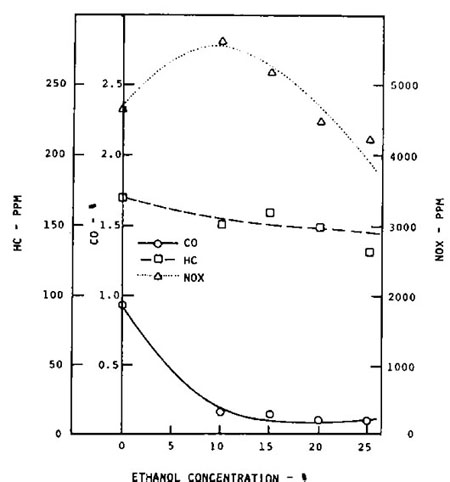
Figure 2: Gasoline engine full throttle exhaust emissions using ethanol fuel blends. |
The effect of using a blend of alcohol and gasoline in an engine adjusted for gasoline is to “lean out” the fuel mixture. This is illustrated in Figure 1 for an engine burning blends of ethanol and gasoline. A mixture of 10 percent ethanol in gasoline produced more power when the carburetor was adjusted for gasoline. Increasing the ethanol content to 25 percent reduced the power output. The test results in Figure 1 were obtained at an elevation of 5,000 feet where an engine adjusted to operate on gasoline is expected to run rich. The 10 percent blend produced a leaner and better air-fuel ratio; the 25 percent blend was too lean.
Because of its higher stoichiometric air-fuel ratio, butyl alcohol can be mixed with gasoline in higher concentrations without affecting performance. Similarly, because of its low stoichiometric air-fuel ratio, only a small quantity of methyl alcohol can be mixed with gasoline without affecting performance. In other words, a fuel blend containing 20 percent methyl alcohol requires modification of the carburetor fuel jets to optimize power output, whereas a 20 percent blend of butyl alcohol does not. In modern flexible fuel vehicles, the engine control unit can use sensors to adjust for different ethanol blends.
The effect on exhaust emissions of increasing the ethanol concentration in the fuel is shown in Figure 2. The primary effect of ethanol is to reduce the CO emissions. It should be noted that the same effect was obtained using straight gasoline and carefully leaning the air-fuel ratio. In addition to CO emission reductions, more recent studies have shown other tailpipe emission benefits such as reductions in emissions of hydrocarbons, oxides of nitrogen (NOx), and certain air toxics. Carbon dioxide (CO2) released when ethanol is used in vehicles may be offset by the CO2 captured when crops used to make the ethanol are grown but the level of offset depends on numerous variables.
Most modern fuel injected vehicles are compatible with gasoline-ethanol blends. Currently, most of the gasoline sold in the U.S. contains up to 10% ethanol (E10) and all auto manufacturers approve blends up to E10 in their gasoline vehicles. As of 2011, the EPA approved a waiver allowing the use of E15 in model year 2001 and newer light-duty gasoline vehicles. Pumps dispensing E15 must be labeled. Consult the U.S. EPA website for specifics on the E15 waiver. E85, also called flex fuel, is an ethanol-gasoline blend containing 51% to 83% ethanol, depending on geography and season. E85 can be used in designated flex fuel vehicles (FFVs) and is available commercially at some filling stations. The vehicle owner’s manual should contain the manufacturer’s maximum recommended ethanol content.
Alcohol and Diesel Engines
Alcohol also has been used in diesel engines. In this case, the alcohol may be blended with diesel fuel to produce diesohol, or the alcohol may be added to the air intake of the engine. A system for adding a mixture of ethanol and water to the air intake of a turbocharged diesel engine is commercially available. The primary function of the system is to cool the turbocharged air (using the latent heat), and thereby to increase the volumetric efficiency of the engine and produce more output power. A similar result can be obtained using an intercooler. Control of the quantity of alcohol added to the air intake may be difficult and could cause erratic engine operation and/or failure if a large quantity of alcohol was added to the air intake.
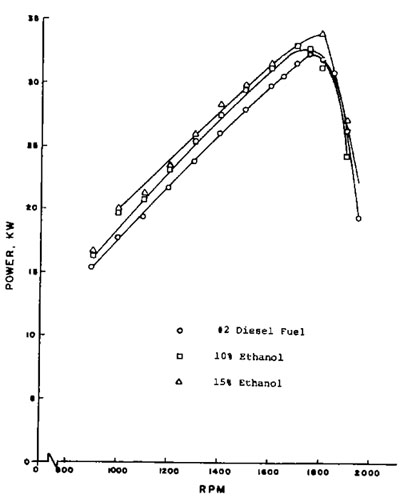
Figure 3: Diesel engine power output using ethanol fuel blends. |
Tests results using blends of ethanol in diesel fuel are shown in Figure 3. The engine used in these tests was naturally aspirated. As with gasohol, the primary effect of the ethanol was to lean the air-fuel mixture and produce more efficient combustion.
Methyl alcohol, because of its highly polar nature, does not mix with diesel fuel. Ethanol can be mixed with diesel fuel provided there is little water in the ethanol. A diesel engine normally will not operate on ethanol nor will ethanol provide lubrication for the fuel injection system. Another problem with adding ethanol to diesel fuel is that the cetane number (ignition characteristic) may decrease below the level recommended by the engine manufacturer.
Corrosiveness
Alcohols may be corrosive to certain materials used in engines. Generally, methyl alcohol is the most corrosive and butyl alcohol is least corrosive. Alcohols also can cause injury or physical harm if not used properly. People who use alcohol in motor fuels should observe warning labels and follow precautions to avoid problems.
*J.L. Smith, former Colorado State University professor and J.P. Workman, former research associate. **A. Drenth, Colorado State University PhD candidate, mechanical engineering; P. Cabot, Colorado State University Extension water resources specialist. 12/04. Revised 11/14.
Go to top of this page.





