by B. Edmunds and L.P. Pottorff * (12/14)
 |
| Figure 1: Gloxinia. |
 |
| Figure 2: Impatiens. |
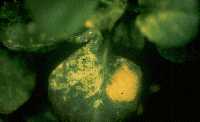 |
| Figure 3: Lobelia. |
Quick Facts…
- Impatiens necrotic spot and tomato spotted wilt virus spread easily throughout the United States because of the way plant material is transported.
- Diagnosis often is misleading, if based on symptoms alone.
- Thrips are common transport vehicles for the viruses.
- Viruses cannot be controlled with pesticides, but instead must be controlled through other management practices. Prevention is best.
- The Colorado State University Plant Diagnostic Clinic can be reached at (970) 491-6950, http://plantclinic.agsci.colostate.
edu/
Plant viruses, as a rule, are named after the first plant on which they are found. Such is the case with tomato spotted wilt (TSWV) and impatiens necrotic spot viruses (INSV, formerly TSWV, I strain).
TSWV first was discovered on tomatoes in 1919 and now is known to infect over 300 different hosts. INSV recently was determined to be a separate virus from TSWV.
Previously, these viruses had been a problem in subtropical areas. It was not until the late 1980s that they began to affect the U.S. greenhouse industry. Because of the way plant material is transported, the viruses spread quickly across the United States and now are found everywhere. TSWV/INSV can cause losses amounting to hundreds of thousands of dollars in a single greenhouse operation.
Some of the more common greenhouse plants susceptible to TSWV/INSV include alstroemeria, begonia, chrysanthemum, cineraria, cyclamen, exacum, geranium, gerbera, gloxinia (Figure 1), impatiens (Figure 2), lobelia (Figure 3), dahlia (Figure 4), ranunculus (Figure 5), petunia and stephanotis. Roses and poinsettias appear to be two of the few plants that do not play host to these diseases.
TSWV/INSV also affect many vegetable crops, such as lettuce, tomato (Figure 6) and pepper, as well as many weed species, such as bindweed and nightshade. The list of annual and perennial ornamental crops affected by this virus is large.
Symptoms
Symptoms of TSWV/INSV vary depending on the host, the environmental conditions affecting the host, and the individual virus infecting the plant. Necrotic spots, streaking, ring spots, stunting and wilting are some of the many symptoms exhibited by these viruses.
Symptoms may resemble fungal and bacterial diseases or environmental stresses. Diagnosis often is difficult, if it is based on symptoms alone.
Transmission
 |
| Figure 4: Dahlia. |
 |
| Figure 5: Ranunculus. |
 |
| Figure 6: Tomato. |
All viruses are obligate parasites, which means they cannot survive outside of their host and must be moved from one plant to another by seed or insects. This process is called vectoring. INSV is carried only by certain species of thrips, the Western flower thrips (Frankliniella occidentalis). Five other thrips species can vector the TSWV, including onion thrips (Thrips tabaci).
The viruses and a thrips vector may be introduced into the greenhouse together on a thrips- or virus-infested plant, or they may arrive in separate shipments. Infected potted-plant crops are an important source of virus for infection of vegetable and flower bedding-plant crops.
TSWV has been reported to be transmitted in the seed of Petunia x hybrida and Lycopersicon esculentum. Seed transmission studies have not yet shown that INSV is spread by seed. It is believed TSWV is carried on the seed coat, rather than in the embryo. Most, if not all, of the spread of these viruses in the North American greenhouse industry appear to be by movement of plants or cuttings, rather than by seed.
To manage these diseases, it is important to know the thrips life cycle and feeding habits, because thrips spend a large part of their lives off the plant. Both virus and vector need to be targeted in control programs.
A thrips completes its life cycle in about 10 days. Eggs are laid in the leaf. Larvae hatch in about three days and immediately begin to feed, thereby picking up the virus. After four days, they pupate in the soil, and in a little over three days, the pupae become adults. Adults feed and transmit the virus.
Only larvae pick up the virus and only adults transmit it. Adults can transmit the virus within 30 minutes of feeding. If larval stages can be controlled, virus transmission can be prevented, even if adult thrips are present (Figure 7).
The amount of time between thrips feeding and the appearance of symptoms varies. It is host and temperature dependent. Symptom expression may occur in less than one week in ‘Calypso’ petunia and within five days in gloxinia. A virus infection also can be latent and symptoms may not appear for months. TSWV/INSV infected New Guinea impatiens show symptoms when grown at 80 degrees F by day and 75 degrees by night. Infected New Guinea impatiens grown at 70 degrees by day, 65 degrees by night, show no symptoms.
Diagnosis
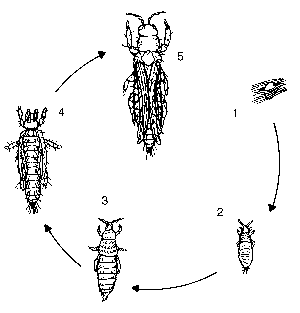 |
| Figure 7: Thrips life cycle.1 = egg 2 = larva (picks up virus) 3 = pupa (non-feeding)4 = pupa (non-feeding) 5 = adult (transmits virus). |
Visual diagnosis of TSWV/INSV is difficult. Symptoms often resemble fungal or bacterial diseases or nutrient deficiencies. Plants may not show any symptoms at all.
An easy, relatively inexpensive, and quick means to detect TSWV/INSV is ELISA, an acronym for enzyme-linked immunosorbent assay. The test uses an antigen/antibody reaction to detect certain virus particles in plant tissue.
Early warning of virus presence also can be noted by using indicator plants or plants that show TSWV/INSV symptoms earlier than others. Such plants include the petunia cultivar ‘Calypso’ and gloxinias. Both plants are extremely susceptible to both viruses and will show symptoms within a week after infection. Non-sticky blue or yellow cards placed near indicator plants can attract thrips and increase the likelihood that the indicator plants will become infested.
For TSWV/INSV testing in Colorado, contact the Plant Diagnostic Clinic at CSU located at E215 Plant Sciences Bldg, Fort Collins, CO 80523-1177, http://plantclinic.agsci.colostate.edu or the Jefferson County Plant Diagnostic Clinic located at 15200 West 6th Avenue, Unit C, Golden, CO 80401-658, 303-271-6620, http://www.extension.colostate.edu/jefferson/hort/diag.shtml.
Management
Viruses cannot be controlled with pesticides. TSWV/INSV can only be controlled with the following management practices.
- Inspection. Inspect all incoming plants for virus symptoms and thrips.
- Isolation. If possible, isolate all incoming plants until certain they are virus and thrips-free (usually seven days is enough). If virus symptoms appear on the plants within four or five days of receipt, it is likely the disease was present when plants were shipped.
- Sanitation. Remove and destroy all infected (symptomatic) plants. They cannot be cured. Destroy all weeds in and around the greenhouse; they can harbor virus and thrips. Do not vegetatively propagate infected plants. To break the disease cycle, avoid continuous cropping.
- Monitoring. Monitor adult populations with yellow or blue sticky cards. Place one to three cards per 1,000 sq. ft. at vents, doorways and at crop height throughout the greenhouse. Check, count thrips and change sticky cards every week. Insecticide treatment often is started when 10 thrips occur per card per week.
Thrips Control
Screening
Since adult thrips are transported by wind, they often enter through vent openings and doorways. Screen these areas to reduce the number of thrips that enter the greenhouse, as well as the amount of TSWV/INSV that is spread. Make sure the greenhouse is clean. Limit access once it is screened. Consult insect screen manufacturers or Colorado State University Extension for proper sizing so air flow is not restricted.
Research at Colorado State suggests that aluminum foil or Aluminet® (reflective shade cloth) screening around vent openings, which changed surface reflectance, decreased migration of thrips through vents by almost two-thirds.
Chemical Control
Thrips have become resistant to many registered insecticides. Use effective chemicals wisely. For registered pesticides, use at least two applications five days apart. Rotate pesticide classes every two life cycles (approximately every three weeks). Always follow label directions and check that products are labeled for the crop you intend to use them on.
| Table 1: Some registered insecticides for thrips control. |
||
| Active Ingredient | Chemical Class | Product |
| Diflubenzuron | Insect growth regulator (IGR) | Adept 25W |
| Novaluron | Insect growth regulator (IGR) | Pedestal |
| Abamectin | Botanical | Avid 0.15EC |
| Azadirachtin | Botanical | Azatin XL, Ornazin3% EC, Aza-Direct |
| Beauvaria bassiana | Fungus (biological) | Botanigard ES, Naturalis-O |
| Spinosad | Microbial | Conserve SC |
| Bifenthrin | Pyrethroid | Attain Greenhouse, Menace GC 7.9% Flowable, OnyxPro,Talstar, Talstar Nursery,Up-Star SC, Wisdom Flowable |
| Fenopropathrin+ Acephate | Pyrethroid | Tame/Orthene TR |
| Bendiocarb | Carbamate | Dycarb |
| Methiocarb | Carbamate | Mesurol |
| Acephate | Organophosphate | Orthene |
| Pyridalyl | Unclassified | Overture 35WP |
Biological Control
Biological control methods can be incorporated into an integrated thrips-management program. They are not a “one time shot.” Use them in an ongoing program along with sanitation, weed control, proper plant culture and screening.
Many thrips insecticides are compatible with biological controls. There
are many beneficial insects available that will manage thrips, including
predatory mites, predatory bugs, beneficial nematods, parasitoids and
pathogens. Predatory mites attack larval thrips, while predatory bugs
attack both larval and adult stages. Beneficial mites and insects can
be purchased from various supply companies in hang-up bags that act as
“mini-insectaries.” For optimum success, note humidity requirements
of biocontrols.
| Table 2: Commercially available biological control agents. |
|
| Predatory mites | Hyopaspis spp., Iphiseius (Amblyseius) degenerans, Neoseiulus (Amblyseius) cucumeris |
| Predatory insects | Orius spp. |
| Parasitoids | Thripobius smilutens |
| Nematodes | Heterohabditis bacteriophora, Steinernema carpocapsae, Steinernema feltiae, Thripinema nickelwoodii |
*B. Edmunds, former Colorado State University Extension regional specialist, commercial greenhouse and nurseries, Adams County. Originally written by Laura Pottorff, former plant pathologist and horticulturist, Integrated Pest Management Program, Jefferson County. 7/96. Reviewed by T. Blunt 12/14.
Colorado State University, U.S. Department of Agriculture and Colorado counties cooperating. Extension programs are available to all without discrimination. No endorsement of products mentioned is intended nor is criticism implied of products not mentioned.
Go to top of this page.





